Metabolism is an essential process to acquire necessary nutrients from outside of cells and utilize them into biomass to maintain cell viability. In a tumor microenvironment that has limited nutrients and oxygen, nutrient availability and nutritional stresses often reprogram metabolic pathways of cancer cells. Hence, cancer cells exhibit distinct metabolic phenotypes to support their survival and proliferation states in tumor microenvironment. Due to the distinct cancer metabolism, blocking primary metabolic pathways of cancer cells could effectively reduce proliferation of cancer cells or inhibit survival of cancer cells, generating cancer vulnerability. To this end, our lab is interested in understanding altered metabolic networks and unveiling regulatory mechanisms of cancer metabolism in solid tumors including breast and head & neck cancer.
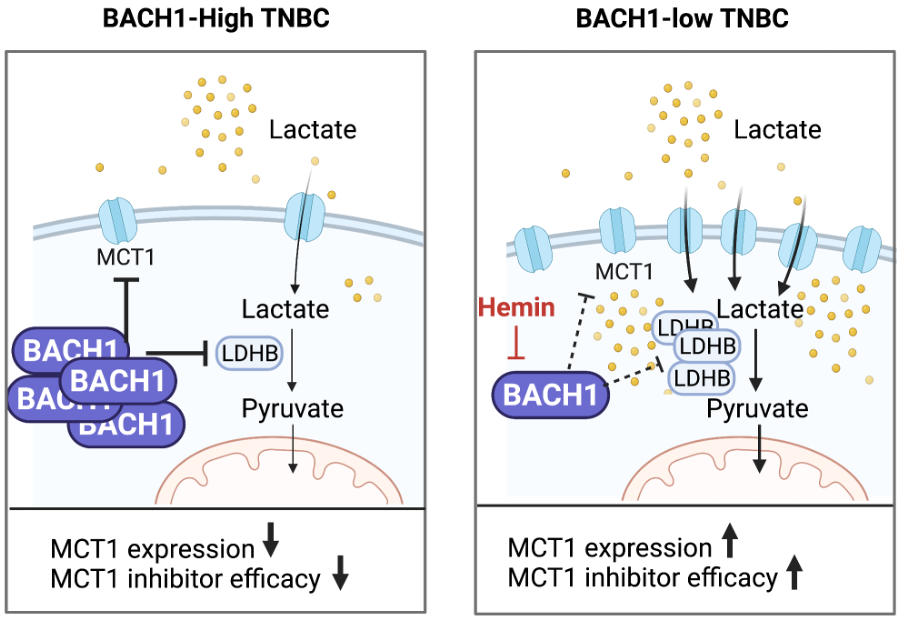
Project I: Lactate catabolism in Triple-negative breast cancer (TNBC)
Cancer cells utilize diverse nutrients depending on oncogenic expression or mutation status of tumor suppressors. Tumor lactate, a byproduct of aerobic glycolysis which is a hallmark for cancer metabolism can be utilized as a substrate for cancer cells, yet its regulatory mechanism of lactate catabolism is not known. Our recent data show that a heme-binding transcription factor BACH1 negatively regulates lactate catabolic pathways in triple negative breast cancer (TNBC), by suppressing expression of monocarboxylate transporter 1 (MCT1) and lactate dehydrogenase B (LDHB) and further lactate-mediated mitochondrial metabolism. Depletion of BACH1 increased lactate catabolism of TNBC cells, rendering cancer cells lactate dependency and sensitivity to the inhibitors targeting MCT1 or LDHB. We will further investigate how to overcome metabolic flexibility and plasticity to reduce metabolic variances among heterogeneous cancer cell populations by targeting a regulator, BACH1. Moreover, we will define molecular mechanism of altered metabolic pathways of breast cancer, asking whether amino acids such as glutamine or fatty acids are involved in lactate-catabolism of breast cancer.
Project II: De novo cholesterol biosynthesis in Head and Neck Cancer (HNC)
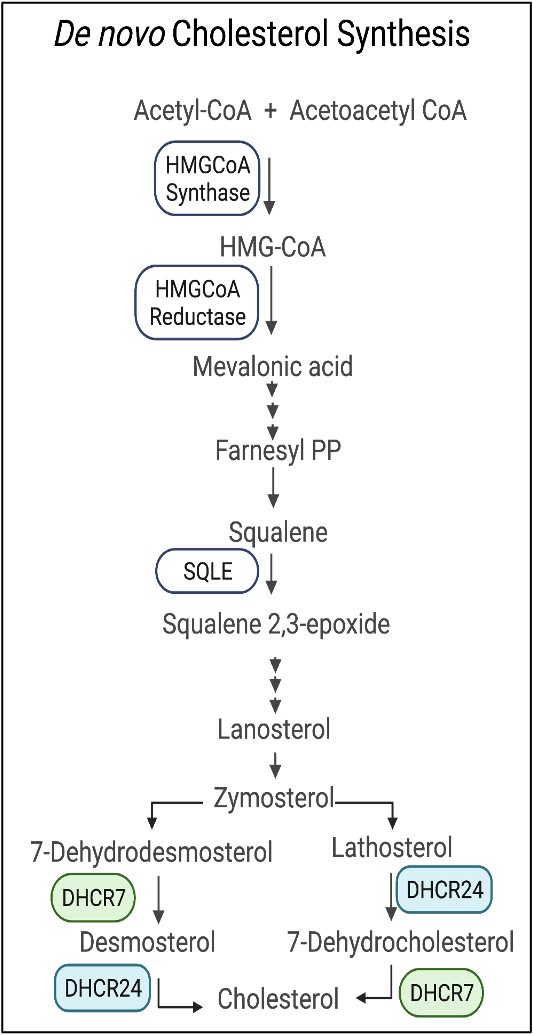
Transcriptomics of head and neck squamous cell carcinoma (HNSCC) tissues from patients (n=19) and their paired normal tissues identified higher expression of genes involved in de novo cholesterol metabolism. Our current research is focused on to understand why HNSCC amplify genes involved in cholesterol metabolism and how to use new metabolic features of HNSCC for cancer vulnerability.
Project III: cMET regulation for metastasis process of Head and neck cancer (HNC)
Activation of c-Met, a receptor tyrosine kinase over-expressed in approximately 80% of HPV-negative HNSCC, enhances proliferation, invasion, migration, metastasis, and angiogenesis. Our lab is interested in cMET signaling and BACH1 induction that promotes cancer metastasis process, and how to interrupt metastasis of HNC.
Project IV: Understanding contralateral breast cancer among young patients with breast cancer
The most common women’s cancer in the world, breast cancer, impacts incidence and mortality among women who then develop a second breast cancer, called metachronous contralateral breast cancer (CBC). The immediate goal of this proposal is to understand the molecular mechanisms by which primary tumors affect contralateral unaffected breast to create CBC. By using mouse models, clinical samples, and cutting-edge technologies, we will ultimately identify risk factors for breast cancer patients for CBC development.